HIGHLIGHTS
- risk management, analysis of applicable regulations and standards, biocompatibility assessment, clinical evaluation
- product testing and certification
- maint enance of our QMS conform requirements of ISO 13485, EU MDR, FDA’s, QMSR, GMP and more
ensuring compliance with regulations and standards.
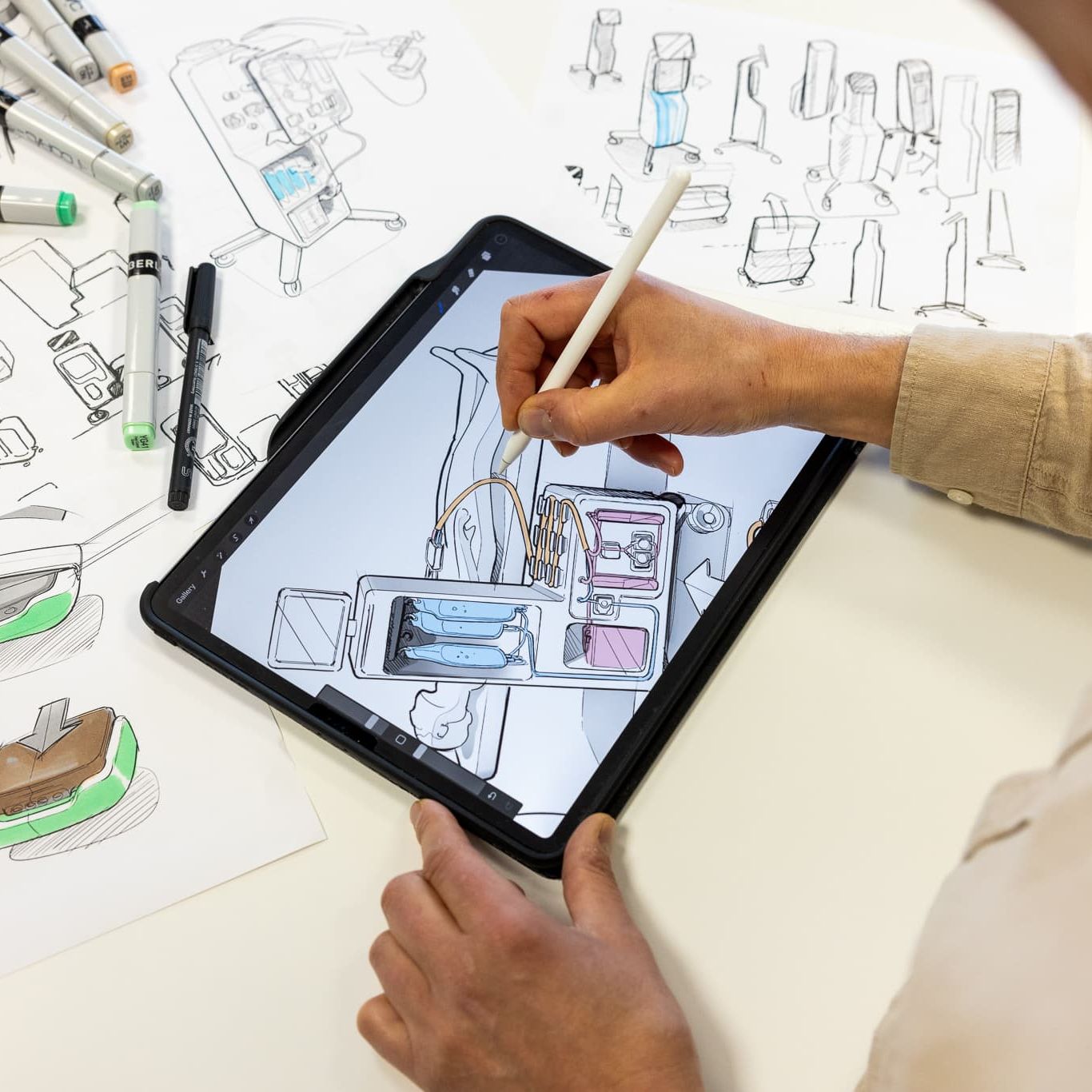
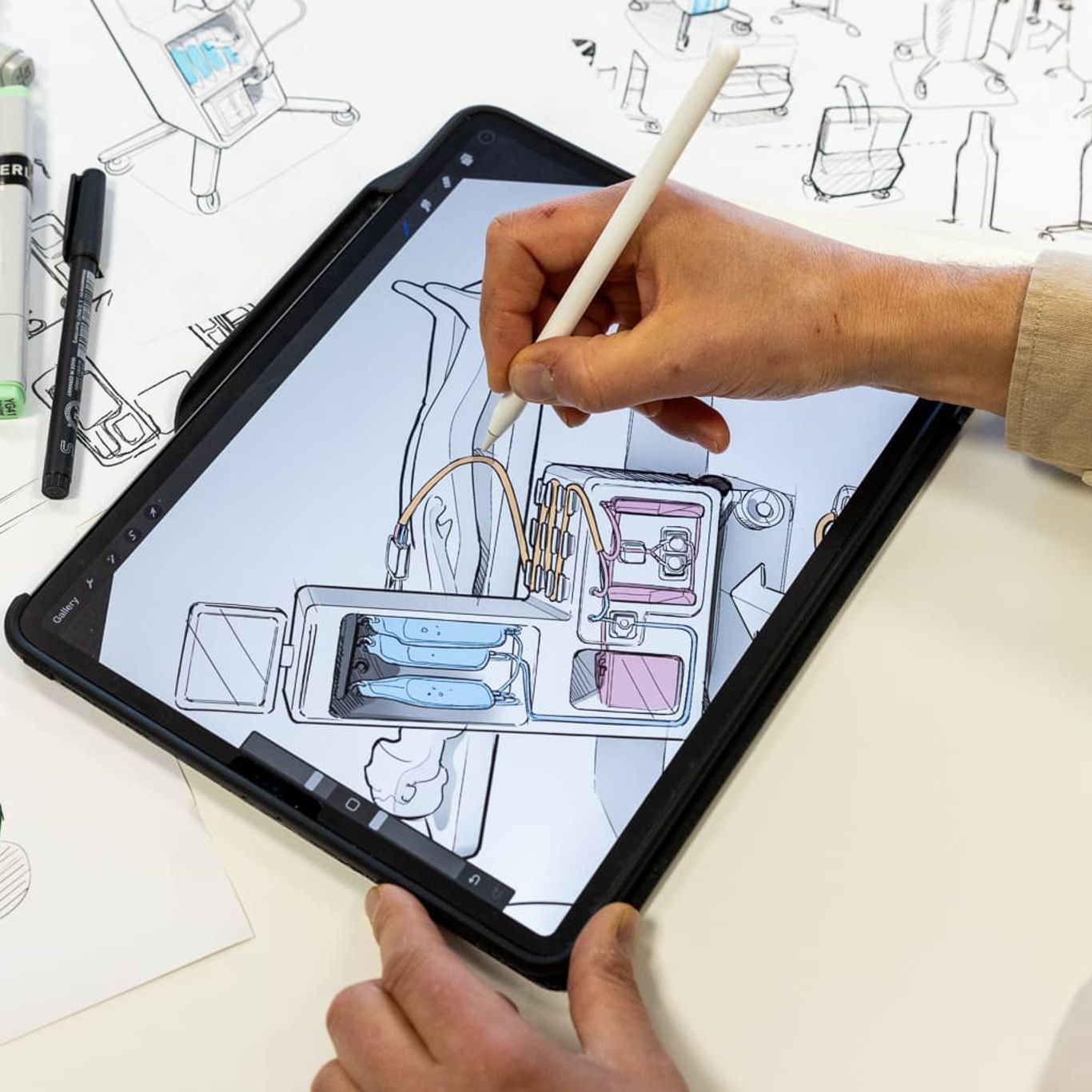
Quality is embedded throughout all project stages—from discovery, concept design and product development to production and certification —making us a true one-stop shop for new product introductions in the life sciences sector. We specialize in medical device and equimpent development, ensuring compliance with the applicable regulations and standards, which are essential for developing safe devices.
expert quality assurance and regulatory affairs for medical devices.
We provide specialized QA/RA services for the medical device and life sciences sectors. Our team is adept at navigating essential regulations, such as the European Medical Device Regulation (EU-MDR) , the European In Vitro Diagnostics Regulation (EU-IVDR ), the U.S. medical device regulation and good manufacturing practice (GMP), ensuring your products meet all compliance requirements.
From the start of each project, we perform a thorough analysis of QA/RA needs to streamline the regulatory process. We can support you in generating and gathering all required evidence, such as risk management, biological assessment and clinical evaluation, to help you achieve certification efficiently. We can assist our customers during the external testing and certification processes by taking an active role in this.
Due to the broad range of products we develop, we are adept to swiftly interpret and implement new standards and regulations, enabling you to stay ahead in a competitive landscape. Our expertise in guiding clients through the certification journey includes medical devices in Europe, the USA and many other regulatory areas , ensuring a smooth path to market for innovative healthcare solutions. With us, you can confidently navigate the regulatory landscape, bringing safe and effective medical devices to patients worldwide.

Demcon-wide certification for quality and compliance.
Our quality management system is certified against various standards that reinforce our commitment to quality, safety and performance, including ISO 13485 for our quality management system and ISO 45001 for maintaining a safe work environment. This comprehensive approach guarantees that our products not only fulfill market needs but also enhance patient safety and ensure regulatory compliance in the medical devices and life sciences fields.