a service that fulfills your needs.
Our services are designed to support both start-ups and established OEMs, with a focus on accelerating the time-to-market for innovative, patient-centered products. As a dedicated development and manufacturing partner, we take ownership of the entire process, collaborating with customers from initial concept through to the final product to ensure efficient and successful outcomes.
contract engineering.
Contract engineering at Demcon life sciences & health enables customers to leverage extensive technical expertise in product development, design, and manufacturing without the need to maintain these resources in-house. By collaborating with us, customers receive specialized support throughout the entire product lifecycle—from initial concept to prototyping, testing, and regulatory compliance. This partnership allows them to focus on their core activities while we accelerate innovation and deliver high-quality, market-ready medical devices.
contract manufacturing.
Contract manufacturing at Demcon life sciences & health enables customers to leverage advanced manufacturing capabilities and technical expertise without the need for in-house facilities. By partnering with Demcon, customers benefit from a full range of services, including scaling up production, supply chain management, and adherence to regulatory requirements. Our state-of-the-art production facilities and commitment to quality ensure the efficient manufacture of complex medical devices, including those requiring precision and reliability.
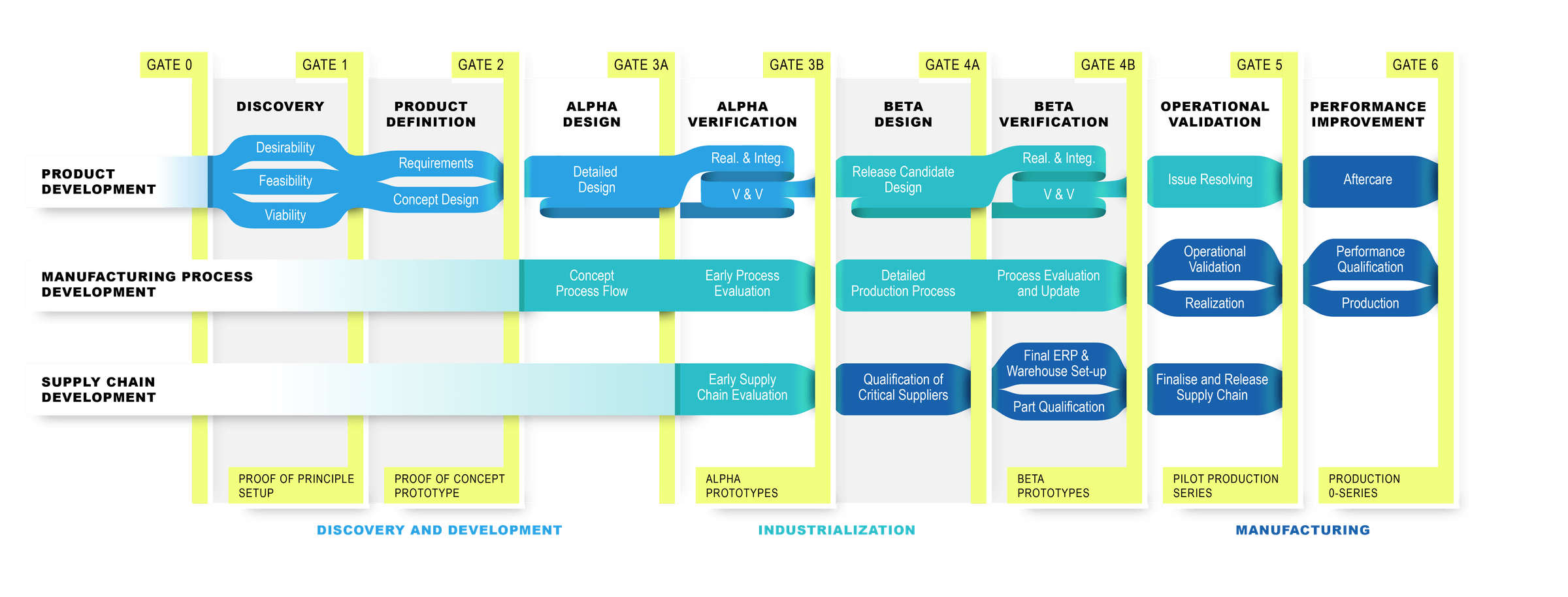
customized stage-gate process for medical device development.
Our stage-gate model is fully adapted to the needs and type of each project, whether in medtech, biotech, or IVD. Each phase is customized and supported by our multidisciplinary teams of experts, ensuring efficient development, expert guidance, and high-quality solutions tailored to your specific requirements. Curious how we support you in your stage of medical device development?
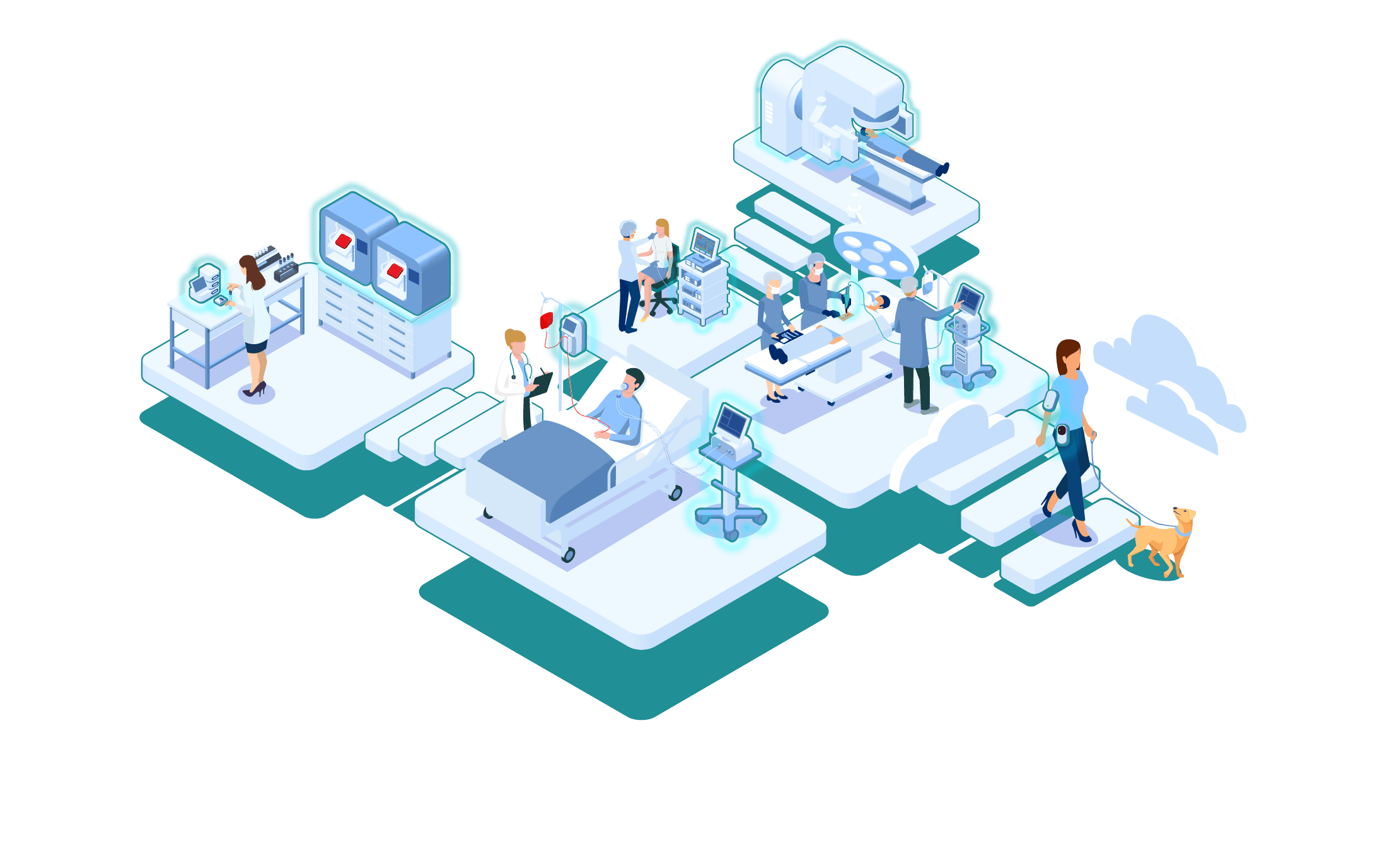
support in every step of the product lifecycle.
Every medical device development project starts with an idea, but bringing it to the next phase can be an issue. Based on our initial meeting, we set up a step-by-step approach to transform the idea into a fully functional, market-ready product using our stage gate model. The general approach is described below, however the approach is tailored to your specific needs to align with the QMS and requested product maturity. Meaning that we can support you in every step depending in which phase you need our support. Every step is tailored to your needs, but to inform you about our stages we included some information below.
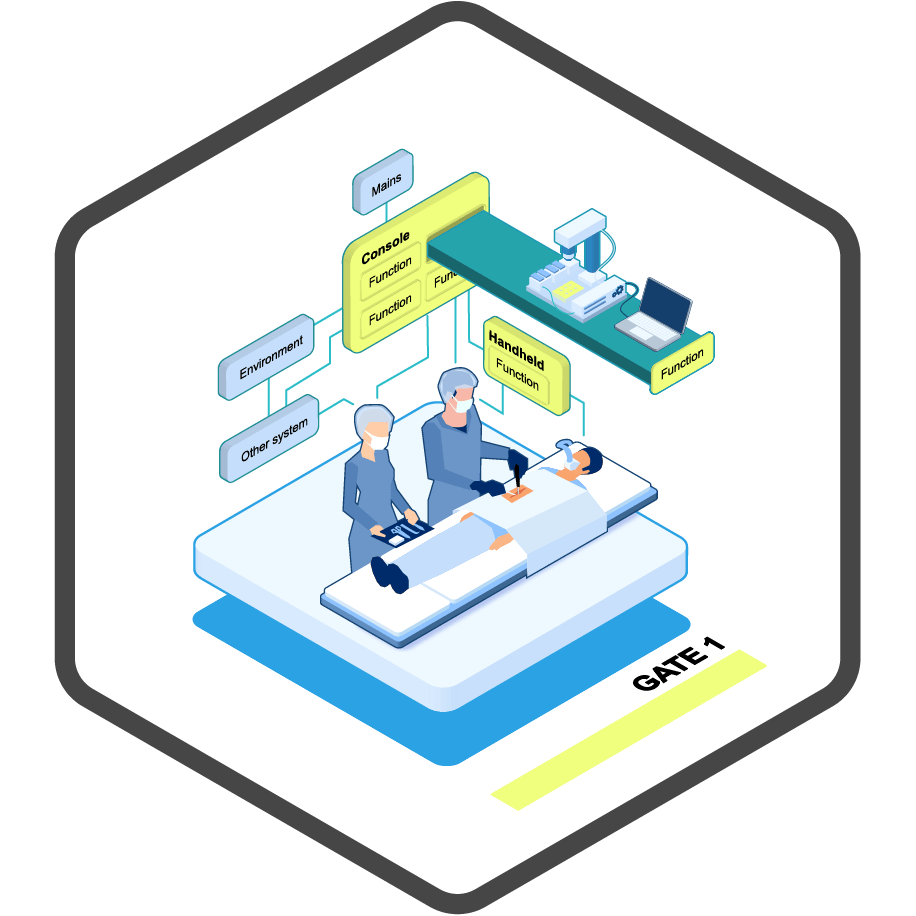
discovery.
Starting with the discovery phase, the idea or concept needs to be shaped. This phase is crucial for the technical feasibility and direction of the project.
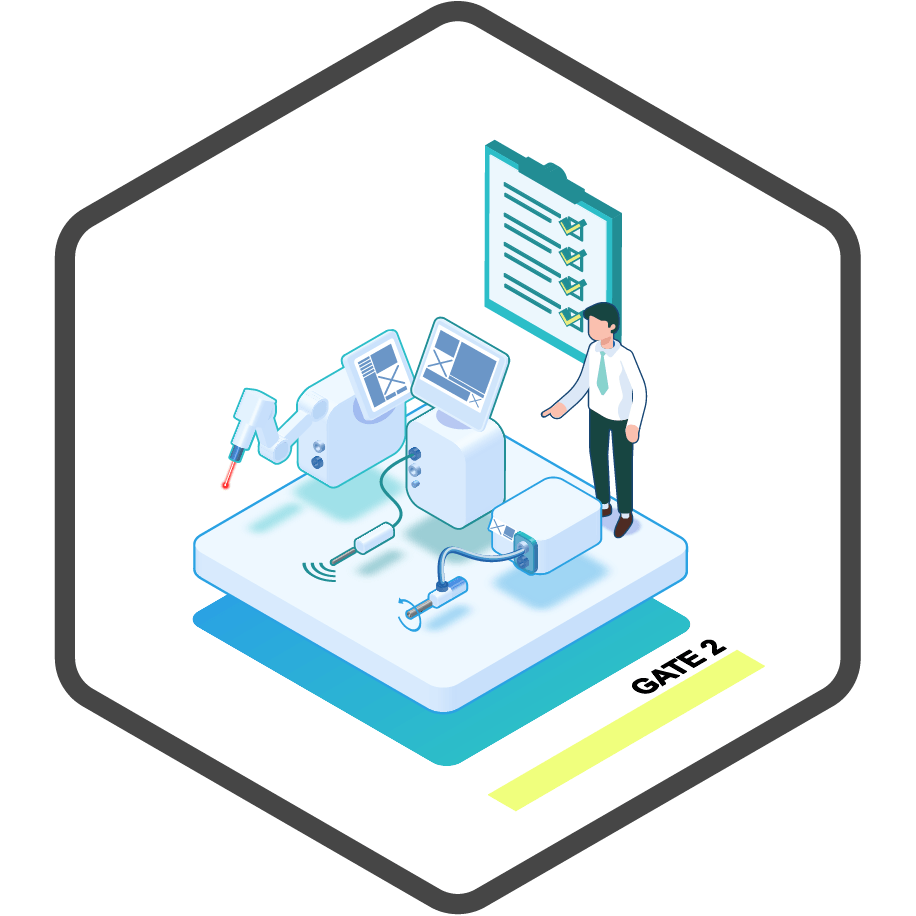
product definition.
This forms the foundation for the product definition phase, where we initiate concept design and begin the initial planning steps. In this phase, we derive system and regulatory requirements and create the product architecture to ensure compliance. Additionally, we conduct early usability tests to align with user expectations and facilitate the path to regulatory approval.
alpha.
Next, we enter the Alpha phase, where detailed design is performed, and the system is realized for the first time. Simultaneously, the initial steps are taken to optimise the design for production. This phase includes system integration, verification, pre-compliance testing, and formative usability testing to ensure alignment with technical and user requirements.
beta.
Following the Alpha phase, we enter the Beta phase, where we refine the design based on Alpha results. The focus shifts toward further industrialization and fine-tuning the production process. During this phase, we assist in finalizing industrial processes, conducting summative evaluations, and selecting suppliers. The Beta system, which closely resembles the final product, undergoes certification tests. Upon successful verification and certification, the product documentation and design are submitted to the notified body for approval.
operational validation.
Once this is set, we support our customers with the final preparations before commercial production and market introduction during the Operational Validation phase. Meaning setting up pilot production runs and validating the production processes (IQ, OC, PQ).
performance improvement.
The last phase of our stage-gate model is the Performance Improvement phase, in which we support our customers with the continuous optimization of both the product and the production process.
high-end in-house facilities.
We provide state-of-the-art facilities for developing and producing high-quality medical products. Our cleanrooms are specifically designed for precision work, while we have dedicated assembly areas to ensure efficient production processes. Our advanced IVD lab enhances testing capabilities, enabling larger project scopes and more efficient execution. We prioritize safety in both development and testing processes. With clear communication and strong partnerships, we ensure seamless transitions from concept to market-ready solutions for our clients.

